Table of Contents
Oracle Clinical Overview
Oracle Clinical Overview and its use in Clinical Data Management
Oracle Clinical Overview – Oracle Clinical provides the life science industry the most integrated Clinical Data Management (CDM) and Remote Data Capture (RDC) application on the market. It was built based on hundreds of companies all having extensive experience in conducting clinical trials thus giving Oracle Clinical an essential industry perspective. In the commercial software marketplace for Clinical Data Management solutions, most vendor offerings consist of separate applications for the design, analysis and workflow processes of Clinical Data Management and the data collection and ease of use characteristics of Remote Data Capture.
Oracle Clinical/RDC provides single application architecture for both CDM and RDC. Oracle Clinical/RDC provides additional functionality in key areas such as integration, data collection, localization, reporting, and especially ease of use.
Features of Oracle Clinical
Oracle Clinical is a comprehensive solution for the management of clinical data. Oracle clinical allows companies to standardize and control data definitions and data usage across a global operation, ensuring that the data is defined, managed and interpreted consistently worldwide.
This accelerates the regulatory process for drug approval and reduces cycle times in the critical data management process. The system is a flexible relational database with the following features:
- A Global Library of reusable objects that permits the fast construction of new clinical studies to exact specifications
- A comprehensive set of tools for capturing the components of the clinical trial protocol in multiple iterations of detail and developmental versions
- A flexible way of structuring the questions that appear on Case Report Forms (CRFs) into database entities called data collection modules (DCMs)
- Automated procedures for generating and customizing data entry windows (forms) that correspond to the CRFs
- Definition of complex data validations and derivations that requires little or no programming
- A discrepancy database automatically synchronized with changes to both the clinical trial data and the validation definitions
- A flexible internal data structure for holding the clinical trial data that provides the ability to reorganize data for extract
- Maintenance of lab reference ranges across multiple studies
Oracle Clinical forms the core component of an integrated suite of applications provided by Oracle under the name Oracle Pharmaceutical applications (OPA). The suite also includes Remote Data Capture (RDC) for entering and managing data from the investigative site, Thesaurus Management System (TMS) for classifying investigator terms against medical dictionaries and the Adverse Event Reporting System (AERS) for managing product safety.
Subsystems of Oracle Clinical
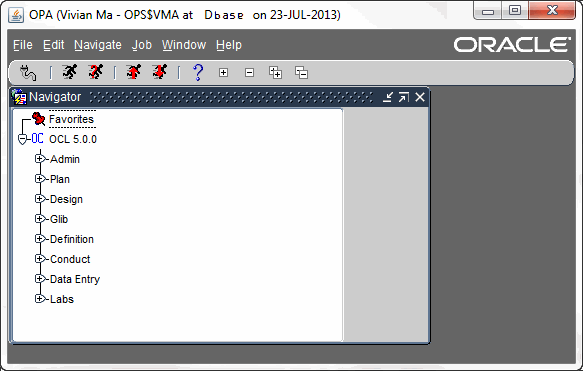
Oracle Clinical consists of several fully integrated subsystems that enable users to set up any kind of study, in any therapeutic area, with any level of complexity in Case Report Form (CRF) design. The key functional subsystems in v 4.5 of Oracle Clinical are:
- Administration
- Plan
- Design
- Global Library
- Definition
- Conduct
- Data Entry
- Labs
However functionally the Oracle Clinical subsystems may be classified under the following categories:
Study Design and Management
This subsystem allows the users to design protocols and amendments, as well as specify how a patient is tracked.
Study Data Definition
This subsystem enables a study to be managed at several worldwide locations concurrently. The essential functions within this subsystem include a global library management, study data definitions, validation and laboratory reference range management.
The global library stores definitions for standard clinical data elements, code lists, CRF data entry layouts, validation and derivation criteria and data extract specifications. The global library is maintained centrally and replicated to all participating sites ensuring that the users can pool the data managed at multiple sites.
The customized study data definitions are standard components from the global library or from prior studies that can be used to quickly set up CRF definitions to define data that will be collected for a new study. Once users have created CRF definitions, default data entry screens are automatically generated and can be modified with a GUI layout editor.
The validation in Oracle Clinical offers a unique facility that dramatically reduces the time users spend defining validations and identifying data problems. This is accomplished through a library of re-usable procedures. Users can define complex cross fields, cross-CRF and cross visit data validations in a non-programmable environment. Many features are included that facilitate typical clinical data manipulations for example average cross visits, baseline vs. endpoint checks, incorporating laboratory ranges etc. The results of these edit checks can be programmed to be ready for investigator review with no manual intervention.
Any new or updated data in Oracle Clinical is checked using the previously defined validations and any discrepancies detected are raised and entered into the discrepancy database.
If a Validation procedure has to be modified it will automatically re-execute. Any newly detected data discrepancies will be raised and existing data discrepancies closed.
This Oracle Clinical subsystem also provides the functionality to manage large number of laboratory normal ranges that can be associated with multi-centric trials. The system allows users to perform the following:
- Define laboratory data and their ranges.
- Manage conversion factors between different units of measure.
- Assign patient data to the appropriate laboratory using a variety of criteria (for example, by site)
- Convert character laboratory data to numeric equivalent (e.g. for urinalysis data or values such as <4.2)
- Access laboratory values alongside their appropriate laboratory ranges when validating or querying data.
- Convert collected data to standard units, including calculation of white blood cell differentials.
Data Capture and Validation
Oracle clinical makes it faster and simpler to obtain clean data and offers the functionality of globally distributed data management. Oracle Clinical allows the same study to be performed multi-centrically without much effort. Study definitions including amendments are automatically propagated to all centers conducting the study. Each centre independently manages its local study sites, including data validation and correction. Oracle Clinical automatically performs validation during data entry. Any unexpected data is accepted and tracked as a discrepancy for later review. It is also possible to create manual discrepancies and enter investigator comments, which are tracked with all other discrepancies.
Recently Oracle Clinical has shifted the scope of data capture from CRF based data entry to Real Time Data Capture through user programs that have the ability to input and update clinical data directly from external real-time sources using the Microsoft C++ API. Thus alternative data capture mechanisms such as bed-side devices, phone based patient enrollment, fax based OCR systems or alternative web based data capture systems can now be directly integrated with Oracle Clinical using the API.
Access and Reporting
Oracle Clinical stores all data in a universal format. This makes study setup, data collection and data extract simple. Additional features include:
- Automatically create views corresponding to each CRF module
- Optionally create custom views combining data from multiple CRF modules
- Automatically extract data into SAS for analysis
- Create any number of data snapshots for interim analysis while normal data processing continues
- Query the data through an online query facility
- Include data (locked/frozen) and discrepancy status information in extracted data.
Oracle clinical supports more than 70 reports to be executed immediately in standard formats such as PDF, HTML, Postscript, RTF or ASCII for review.
Other major component of Oracle Clinical include Integrated Discrepancy Management System which automates the entire error correction process by maintaining and online record of all data problems detected by the system or identified manually. This enables the discrepancy review to occur online with the ability to generate and correct problems. If the discrepancies are to be communicated to the investigator using query forms, this subsystem of Oracle Clinical provides the capability for grouping discrepancies onto Data Clarification Forms (DCFs), printing the forms in a configurable report format and tracking the receipt of the forms once completed. Oracle Clinical keeps a full audit trail of both the data and the discrepancies and this audit trail is available online and through printed reports.
Another component of Oracle Clinical is the Thesaurus Management System (TMS). This component enables medical or drug terms to be automatically encoded during the validation process by referencing industry standard dictionaries such as MedDRA, COSTART, WHO-ART, WHO-DRL etc as well as any in-house dictionaries.
The most recent version of Oracle Clinical 5.0.1 has the following subsystems which are nothing but further classifications to the previous versions. These are Administration, Planning, Lab Reference ranges, Global Library, Design, Data definition, Conduct and Data extract.
This completes our discussion on Oracle Clinical Overview. We hope this helps you get an insight into Oracle Clinical Overview.
For a deep insight into the world of Clinical Data Management, subscribe to our Clinical Data Management Knowledgebase
Want a explore a career in Clinical Data Management? Join our Diploma in Clinical Data Management program and kick-start a career in Clinical Data Management and Oracle Clinical.
Already completed a program in clinical data management. Enhance your expertise on the Oracle Clinical software by pursuing our Oracle Clinical Fundamentals program. You can also subscribe for 24×7 access to the Oracle Clinical software for practice.