This post is a Guide to Narrative writing that will help you understand the importance and various elements of Narrative writing.
Table of Contents
- Introduction
- Basic Terminology
- Narrative Writing Principles
- Rules of Narrative Writing
- Checking a written Narrative
- Writing Process Flow chart
- Glossary
- You may be interested in…
- Oracle Argus Safety Essentials
- Oracle Argus Safety Essentials + Console
- Oracle Argus Safety – Live Online
- Oracle Argus Safety + Console – Live Online
- Oracle Empirica Signal
- Oracle Empirica Signal – Live Online
- Diploma in Pharmacovigilance
- Argus Safety – Business Configuration and Administration
Introduction
The idea of this guide is to introduce the newcomers to the basic writing principles. This is based on the initial writing experiences of other newcomers and the initial difficulties faced by them.
Basic Terminology
Some of the basic terms a medical writer comes across are explained below.
Clinical Trial: These are the trials companies do to test the efficacy of their drug as well as to look for adverse effects before getting approval for launch in the open market. There are several phases (Phase 1 to 4) in which they are conducted. More details can be looked at appropriate places.
Source Files or CRF: Whenever a trial goes on, the records of a particular subject are compiled together on a day to day basis throughout the trial and these are called source files or CRF (case report file). It is from these source files (called appropriately so) information is extracted while writing a narrative.
CIOMS: This is the “Council for International Organizations of Medical Sciences”, was formed in 1949 by WHO and UNESCO. CIOMS form is the standard reporting format of serious adverse events while conducting clinical trials worldwide to respective regulatory authorities.
SOP: This is Standard Operating Protocol. Once finalized, this is the gold standard to be stuck to, in letter and in spirit, while writing a narrative. It defines exactly how the narrative will be written.
Narratives: Narratives are synopsis of a particular subject who has been involved in a clinical trial. It may be of any phase and anywhere in the world. If a source file is very well documented almost all the information needed for writing a narrative can be found here. In case there is something missing CIOMS report is also a narrative in itself and can give good amount of information.
Narrative Writing Principles
This section explains how to frame the complete narrative.
Writing Style: This is determined by the choice of the client but there exists a certain format in which all narratives. Please have a close look at the following sequence of statements that are followed in a narrative.
Narrative Outline: A narrative is written with these sentences coming one after another in the following order. Please give special attention to the order of these sentences.
1) Subject ID (most serious adverse event/events):
2) This age-year-old ethnicity man/woman had a medical history of XXXXX. Including past history and history at the time of the start of the trial.
3) Upon study admission, the subject was noted to have XXXXX. The reason he/she is being treated with the study drug.
4) He/She was randomly assigned to treatment with XXXXX. Study drug and dosing regimen,
5) A description of the most serious adverse event (e.g. prolonged hospitalization or death) including the day it started, its effect and the action taken (e.g. withdrawal of treatment or change in dosing regimen) if any.
6) A description of the nature (intensity) and relationship of this event to the study medication, e.g., not related/possibly related/related to the study medication. (causality information)
7) The treatment used for this event and the outcome (resolved, persisted, etc.) If death was the most serious event, this sentence is not necessary
8) Other adverse events are listed and relation of these adverse events and their outcomes are given too.
9) List and description of laboratory results/vital signs which are related to the serious adverse event/events and their outcome and/or a statement stating that no significant lab values/vital signs were reported.
10)The last sentence as statement, “The subject completed the study on Day X or The study was terminated on Day X due to XXXXX”.
Now look at the following narrative and note all these ten sentences forming the complete body of narrative. The sentence number is superscripted in red color after it has been placed in box parenthesis.
[Subject ### (infection of both wrists after suicide attempt, left wrist cellulitis, and suicide attempt)]1: [This 21-year-old Caucasian woman had a history of alcohol use, amphetamine use, heroine use, depression, mitral valve thickening, and right eye blindness.]2 [Upon study admission, the subject was noted to have abscess that was positive for Staphylococcus aureus.]3 [She was randomly assigned to treatment with ceftobiprole, 500mg twice daily administered intravenously over 60 minutes.]4 [The subject experienced serious adverse events, infection of both wrists after suicide attempt (Day 27) and left wrist cellulitis (Day 31).]5 [All the serious adverse events were life threatening and considered unrelated to study medication.]6 [The subject was treated with psychiatric counseling and pip/tazo, and the events resolved without sequelae.]7 [Other adverse events reported during the study were mild butterscotch aftertaste (Day 1), which was considered possibly related to study medication, and mild left arm paresthesia (Day31), which was considered unrelated to study medication. Both events resolved without sequelae.]8 [No clinically significant changes in laboratory values, vital signs, or physical examination results were reported during the study.]9 [The subject completed the study on Day 42.]10
Following is the list of sections of source file where a newcomer can look for information again following the same order of sentences.
- Demographic Profile and Adverse Events
- Medical History
- Medical History and CIOMS Report
- Dosing section, CIOMS and SOP (this statement is generally decided as it is a standard statement to be used in all narratives
- Adverse Events and CIOMS
- Causality Column of Adverse Event Section
- ConMed Section
- Adverse Event Section
- Laboratory Section and Vital Sign Section
- Study Comments Section. Read its different columns carefully for complete information.
Guidelines for Writing a Narrative:
- The Header: This is the first line and written in bold. It includes subject XX and then the,
-
- Adverse event which led to death, or
- Adverse event which led to discontinuation of study, or
- The serious adverse event.
If there are more than one serious adverse event, all should be included.
2. Medical History and ConMeds: Only the medical history which is relevant in terms of the SAE should be considered. Same is the case of concomitant medications. Needless elaboration should be avoided.
3. Study Drug: The drug, dose and duration to be mentioned in a statement.
4. Serious Adverse Event: This is the soul of a narrative and the term to be used is the MedDRA (or WHOART) preferred one but investigator’s term may be used to clarify further in parenthesis. Following information about SAE should always be provided,
- Timing of SAE
- Diagnostic Procedure if any
- Nature and Intensity
- Causality Information according to the investigator
- Action taken for the SAE, like, stopping the drug, etc.
- Outcome of SAE
5. Other Adverse Events: Only those AEs which are related to the SAE should be mentioned. Same information should be provided for AEs as for SAE.
6. Laboratory findings and Vital signs: Once again only those which are related to the SAE should be taken into account.
7. CIOMS: Whenever information comes from CIOMS report, CIOMS should be mentioned in parenthesis at the end of information. Mention the complete CIOMS report number at the end.
Organizing Written Narratives:
Narratives are organized in batches. They are divided according to
- Study group and then
- According to their number.
They are further sub categorized according to,
- Death
- SAE
- AE leading to discontinuation
If there is a subject who falls in more than one of the three categories, a single narrative is provided for him/her and the order to be followed is the same as above, i.e., death, SAE and AE leading to discontinuation.
Sometimes the client will want multiple narratives for the same subject. In this case the major narrative is the one involving the most serious category. Then there could be minor narratives for the less serious categories. DEATH might be the major and SAE and DC would be minor narratives.
Rules of Narrative Writing
1. Style: The writing style of all narratives of a clinical trial must be similar.
2. Grammar: This must be impeccable. There is absolutely no scope of wrong English as this defeats the whole purpose of good writing.
3. Spellings: It may be noted that generally there are two types of Spellings and they are American and English. Narratives are as a rule written according to clients’ wishes and it will depend where the company is based. Confirm the spelling style from SOP.
4. Spacing: There is always one space between words and either one (English style) or two (American Style) after a period. Confirm from SOP what the client wants.
5. Hard Spaces (hard hyphens also):
How to put: This is placed by ctrl+shift+space.
The concept of hard Space: Its concept has to be understood and then only its importance can be realized. A normal space can split two words connected by it in two lines when it is opened in computer; but a hard space, which looks exactly like a normal space, keeps these two words connected in whatever format or program that document is opened. Thus it serves a very important purpose.
Where to apply: It should be applied at following spaces,
-
- Between Day and its number
- Between a Lab test and its value
- Between the subject and its ID no.
- Anywhere else where we want to connect two words/values inseparably.
6. CIOMS: Whenever some information is directly taken from CIOMS report, place CIOMS in parenthesis after that.
Checking a written Narrative
This is required when one’s own narrative is checked or if one is asked to QC (Quality Check) someone else’s narrative. One can form any order but a simple outline is given below,
- Check if the language and style of narrative is SOP compliant.
- Check the spellings and grammar. For this Word is not very useful as it doesn’t know most of the medical terms. This has to be done manually. Check what the client wants, i.e., whether English or American spellings and period pattern. Proper use of comma, period, colon, semi-colon and dash is a must.
- Check for accuracy of content from source file or CIOMS report and also if all the information as per SOP is given.
- Check specially for small dash (or minus sign) which is automatically converted to a big Dash when we type, i.e.; when we type, e.g., Day space dash X space the dash will become big Dash. Convert it to small Dash or minus sign as the case may be.(Client specific requirements for Dash, Hyphen, Hard hyphen, Em dash, or En dash)
- Finally Check for Spaces. Turn the space button (denoted by space or music notation sign, just left to your zoom button on the MS-Word Toolbar) on and look at the text. All normal spaces are shown by a “Dot” while all hard spaces will be shown by a “Small Circle”. Now correct them as advised earlier. Check the sample narrative given above by clicking space button to understand this.
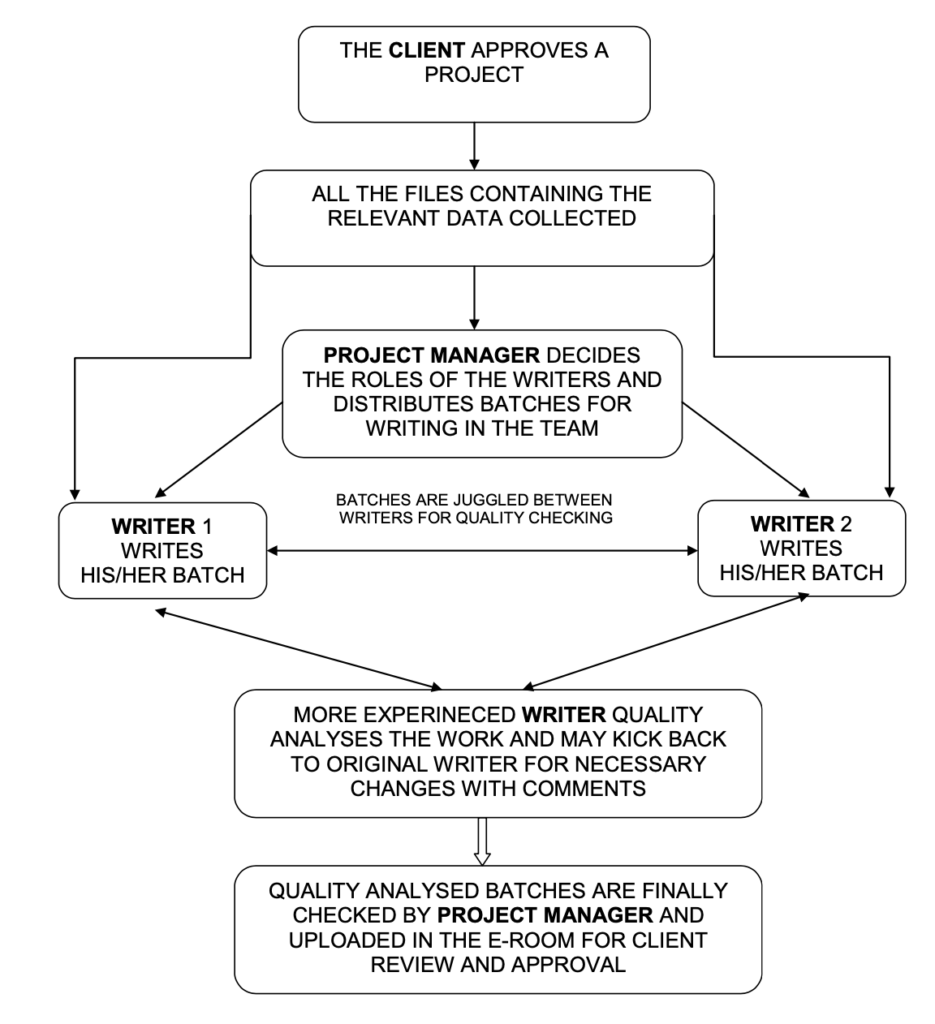
Writing Process Flow chart
Glossary
This is a list of very commonly used terms.
AE – Adverse Event
CIOMS Form/Report – Serious Adverse Event reporting form ConMed – Concomitant Medication
CRF – Case Report File
EOT – End of Treatment
QA – Quality Analyze
QC – Quality Check
ReQC – Repeat Quality Check
SAE – Serious Adverse Event
SOP – Standard Operating Protocol
TOC – Test of cure
This completes our discussion on Guide to Narrative Writing. We hope this gives you flavour of the Guide to Narrative Writing and how it is used in pharmacovigilance.
For a deep insight into the world of Pharmacovigilance, subscribe to our Pharmacovigilance Knowledgebase Want to explore a career in Pharmacovigilance? Join our Diploma in Pharmacovigilance program and kick-start a career in Pharmacovigilance and Oracle Argus Safety.
Already completed a program in Pharmacovigilance. Enhance your expertise on the Oracle Argus Safety software by pursuing our Oracle Argus Safety program. You can also subscribe for 24×7 access to the Oracle Argus Safety software for practice.
You may be interested in…
-
 eLearning + software
eLearning + softwareOracle Argus Safety Essentials
$599.00 -
 eLearning + software
eLearning + softwareOracle Argus Safety Essentials + Console
$799.00 -
 Live Online
Live OnlineOracle Argus Safety – Live Online
$999.00 -
 Live Online
Live OnlineOracle Argus Safety + Console – Live Online
$999.00 -
 eLearning + software
eLearning + softwareOracle Empirica Signal
$599.00 -
 Live Online
Live OnlineOracle Empirica Signal – Live Online
$999.00 -
 eLearning + software
eLearning + softwareDiploma in Pharmacovigilance
$799.00 -
 eLearning + software
eLearning + softwareArgus Safety – Business Configuration and Administration
$599.00