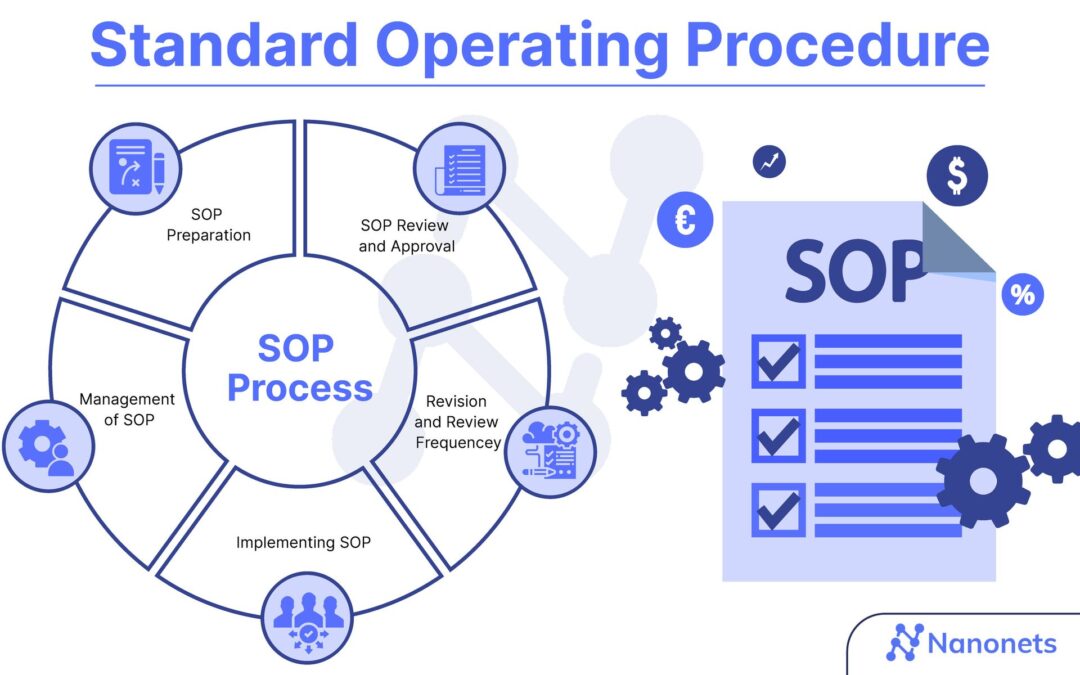
by ClinSkill | Feb 16, 2023 | Clinical Data Management, Oracle Clinical
SOP for Data Entry in Oracle Clinical A SOP (Standard Operating Procedure) in clinical trials is a documented set of procedures and instructions that describes how specific tasks or activities are to be carried out in a clinical trial. It provides a standardized,...

by ClinSkill | Jul 1, 2022 | Clinical Data Management, Oracle Clinical
Oracle Clinical and RDC upgraded to 5.4.0 We have been offering Oracle Clinical and RDC 5.0.1 software access as part of our Clinical Data Management and Oracle Clinical courses, as well as through our Oracle Clinical – software access subscription service. This...
by ClinSkill | Apr 16, 2018 | Clinical Data Management, Oracle Clinical
Electronic Data Capture is one of the new technologies have impacted the process of clinical data management and have been well accepted to ensure a more efficient and effective method of data collection. Electronic Data Capture Overview The CDISC defines Electronic...

by ClinSkill | Mar 5, 2018 | Clinical Data Management, Oracle Clinical
Clinical Data Management – Processes during Data Collection Clinical Data Management – Processes during Data Collection. Once the CDMS has been installed and the configured and after the systems have been validated, the process of data collection can...

by ClinSkill | Mar 1, 2018 | Clinical Data Management, Oracle Clinical
Clinical Data Management – Processes Before Data Collection Clinical Data Management – Processes Before Data Collection. A good data management method involves the defining of processes for collection of data and its management even before we begin to...
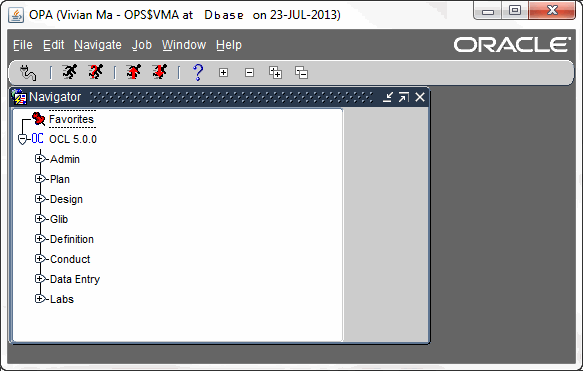
by ClinSkill | Feb 8, 2018 | Clinical Data Management, Oracle Clinical
Oracle Clinical Overview Oracle Clinical Overview and its use in Clinical Data Management Oracle Clinical Overview – Oracle Clinical provides the life science industry the most integrated Clinical Data Management (CDM) and Remote Data Capture (RDC) application...







