
by ClinSkill | Jul 5, 2023 | Clinical Research
SOP for Clinical Research Management Plan Purpose: The purpose of this Standard Operating Procedure (SOP) is to provide guidelines and instructions for the development and implementation of a clinical research management plan. This plan ensures the efficient and...

by ClinSkill | Nov 25, 2022 | Clinical Research
Principles of Good Clinical Practices Principles of Good Clinical Practices – Role of the FDA In this post we will discuss about the basic principles of Good Clinical Practices and how they apply to clinical trials. Before discussing the Good Clinical Practice...

by ClinSkill | Dec 12, 2019 | Clinical Research, Oracle Siebel Clinical
In this post we will be providing an overview of Oracle Siebel Clinical and how organisations use Siebel clinical to manage their clinical trials more efficiently and effectively. Learning Objectives – Oracle Siebel Clinical By the end of this post, you should...

by ClinSkill | Feb 26, 2018 | Clinical Research
Regulation Governing Computer Systems in Clinical Trials Introduction – Regulations Governing Computer Systems in Clinical Trials Regulation Governing Computer Systems in Clinical Trials. The FDA considers the pharmaceutical companies that conduct trials to be...
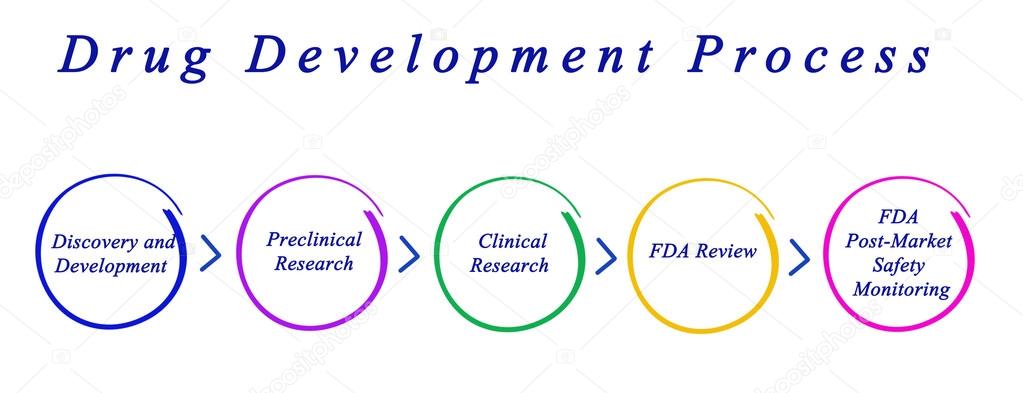
by ClinSkill | Jan 18, 2018 | Clinical Research
The Drug Development Process Once a compound (Drug candidate) is selected and patented, so as to secure space for innovation within the fast moving research arena, the compound has to undergo several stages of preclinical (animal) testing and clinical (human) testing...

by ClinSkill | Jan 12, 2018 | Clinical Research
Introduction to Drug Discovery Drug discovery is a research, but should not be confused with academic research. Most academic research involves researching on something for the sake of understanding and there is no final goal. However drug discovery has a focused...





